Student Spotlight: Discoveries from university researchers
By Mariia Usiichuk, Vasylyna Kirianchuk, Oleg Shevchuk, Tetiana Shevtsova, and Andriy Voronov
The search for alternatives to petroleum-based materials in the polymer industry, driven by growing environmental concerns and limiting the availability of petroleum-based sources, is becoming increasingly critical. In this context, plant oils, with their renewable, biodegradable, and eco-friendly nature, have proven to be promising raw materials.1 Recently, various approaches, including acrylation, epoxidation, and transesterification, have been developed to transform the triglycerides present in oils into polymerizable monomers, paving the way for the creation of biobased polymers with properties comparable to their petroleum-based analogs.2 The flexibility in chemical modification enhances the potential applications of plant oil-derived monomers in various industries, including coatings.3
Coconut oil, in particular, is an attractive source for biobased monomer synthesis due to its unique composition, rich in medium (6 to 12 carbon atoms) saturated fatty acids such as lauric acid.4 This composition makes coconut oil highly valuable for producing additives, such as surfactants, where the shorter-chain fatty acids enhance key performance properties. However, the conversion of hydrogenated oils into surfactants typically involves multiple processing steps, which adds complexity to the overall production process.5
Our research group has developed an efficient one-step method to convert triglycerides from plant/vegetable oils into a library of plant oil-based acrylic monomers (POBMs), suitable for free radical polymerization.6,7,8 It was shown that the incorporation of POBMs made from primarily unsaturated triglycerides provides several benefits to the resulting polymeric materials, including a plasticizing effect and controlled viscoelastic behavior. At the same time, plant oil-based monomeric fragments from saturated fatty acids in the oils facilitate the formation of ordered (crystalline) morphological polymeric domains. Such combination enables tailoring of the thermo-mechanical properties in a broad range and thus advances the performance of POBM-based polymeric materials. In this short communication, we elaborate on the highly saturated triglycerides effect by introducing recently synthesized coconut oil-based acrylic monomer (CCM) to broaden our technology of fabrication of biobased polymeric materials with tunable properties.
Synthesis and Characterization of Coconut Oil-based Acrylic Monomer
The composition of the coconut oil used as raw material was analyzed by mass spectrometry in order to assess the feasibility of monomer synthesis and relate the composition to the characteristics of the resulting polymers. The results indicated that the coconut oil contains over 90% saturated fatty acids (Table 1), which aligns well with existing literature.4 Additionally, the low iodine value 16 g/100 g found by titration confirms the saturated nature of used coconut oil.

The coconut oil was then converted into coconut oil-based acrylic monomer (CCM) using the one-step transesterification method described in our previous publications.6,7,8 As shown in Figure 1, coconut oil was reacted with N-(hydroxyethyl) acrylamide, yielding a mixture of monomers that feature a single acrylic double bond linked to fatty chain residues, where R1, R2, and R3 represent fatty acid fragments corresponding to the oil chemical composition. The resulting product was purified according to the established methodology and then characterized.
FIGURE 1 The mechanism of the transesterification reaction.
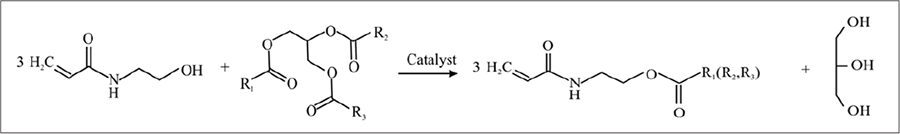
The structure of the CCM was confirmed using FTIR and 1H NMR spectroscopy (Figure 2). The FTIR spectrum indicates the incorporation of fatty acid acyl moieties to the acrylamide fragment. The presence of a strong NH absorption band between 3200 and 3400 cm–1, along with the carbonyl (amide I) band at 1670 cm–1 and the NH (amide II) band at 1540 cm–1, confirms the bonding of the acrylamide group to the fatty acid fragment. Additionally, the presence of strong absorption bands observed at 1740, 1245, and 1180 cm–1 confirms the ester nature of the CCM (Figure 2A).
According to the 1H-NMR spectrum (Figure 2B) of the CCM, characteristic peaks for the acrylic double bond protons are observed at 5.6-6.6 ppm along with the peaks corresponding to the protons of the ethylene linkage between the amide and ester groups (3.6 ppm and 4.20 ppm), and signals from the fatty acid chains (0.8 and 2.8 ppm), confirming the monomer structure, which includes both acryloylamide and fatty acid moieties.
FIGURE 2 FTIR (A) and 1H-NMR (B) spectra of CCM.
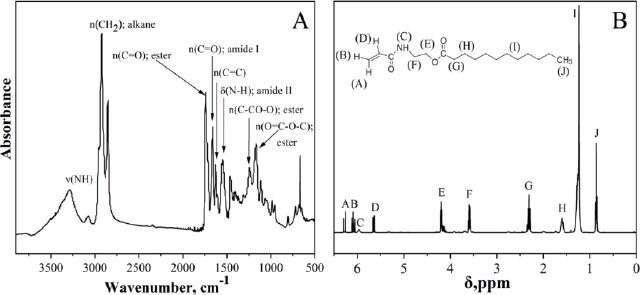
To confirm the structure of CCM, Electrospray Ionization Mass Spectrometry (ESI-MS) was used (Figure 3), and the presence of the predominant [laurate-CCM + Na]+ fraction was confirmed, with the highest peak observed at 320 m/z. These results correlate with the fatty acid composition of the coconut oil used for synthesis, where lauric acid fragments comprise approximately 60% by weight. Additionally, the CCM molecules also contain smaller fractions of other fatty acid chains, as indicated by mass peaks at 264, 348, 376, and 402 m/z. Given the diverse fatty acid composition of coconut oil, the CCM is a mixture of monomers, with lauric acid residue being the most prevalent.
FIGURE 3 ESI mass spectra of CCM.
The iodine value of CCM (65 g/100 g) is higher than that of coconut oil due to the presence of acrylamide moiety, yet as it can be expected, this value is significantly lower than that of unsaturated plant oil-based monomers (POBMs) reported previously by our group.7
Continue reading in the November-December digital issue of CoatingsTech
